Data Access

Collaboration and Access
The GARBH-INi Data Dashboard offers researchers an overview of available data and detailed guidelines for requesting access. By providing de-identified datasets, the platform ensures participant confidentiality while facilitating collaborative research to advance maternal and child health.
Submit a Request for Access
Researchers seeking access to GARBH-INi data and sample can follow the dashboard’s guidelines to submit a request. Access is granted to support ethical and impactful research aimed at advancing maternal and child.
The dataset includes comprehensive participant information, such as demographic, clinical, and ultrasound records, collected at multiple visits with biospecimens to ensure robust longitudinal insights.
The dataset includes comprehensive participant information, such as demographic, clinical, and ultrasound records, collected at multiple visits with biospecimens to ensure robust longitudinal insights.

What Will You Need?
To streamline the registration process for accessing GARBH-INi data, please ensure you have the following documents and information ready before you begin:
- Up-to-date Résumé/CV: Your CV should verify your current position and institutional affiliation in English.
- Publication References: Provide PubMed reference numbers of up to 5 peer-reviewed publications in which you are named (if available), or a link to your personal profile on your institution's website.
- Institutional Email Account: Use an email specific to you and your affiliated institute.
- Failure to provide the required information may result in delays or rejection of your registration.
Confirming Your Institute
When completing your registration form, you will need to select your institute from the approved list. If your institute is not listed, you will need to provide its details for approval.
To approve an Institute/Academic Organization, we require:
When completing your registration form, you will need to select your institute from the approved list. If your institute is not listed, you will need to provide its details for approval.
- Evidence that the institute is a legitimate research entity with prior experience in health-related research.
- A website confirming the nature of the institute, its location, and its affiliated email domain.
- The legal entity name of the institute as it should appear on official documents.
Activating Your Account
Once your registration is submitted, remember to activate your account. Check your email for an automated validation link, which must be activated before your registration can be reviewed. Having all required documents and completing each step carefully will ensure a smooth and efficient registration process
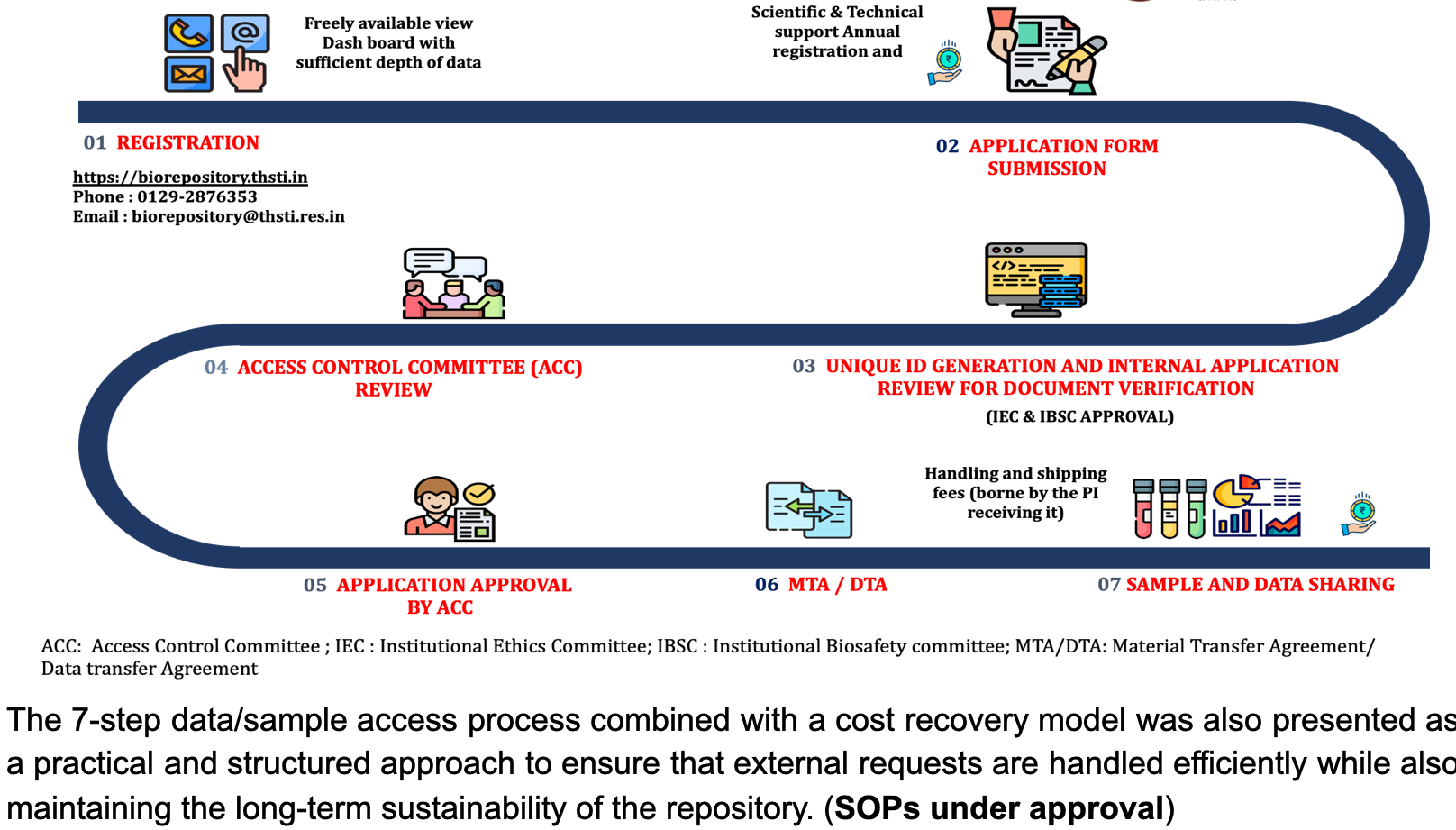
Step by step procedure for Access to and Sharing of Biospecimen and Associated Data
The approval for all requests from external PIs, will be routed through a DBT-nominated GARBH-Ini Access Control Committee (GACC), consisting of esteemed members from the funding body, prestigious institutions, including domain experts.
- Step 1: REGISTRATION BY THE USER: Provide necessary information such as name, organization name, email & contact number along with brief CV with publication details and pay annual registration and scientific and technical support fee followed by REGISTRATION APPROVAL AND USER LOGIN.
- Step 2: APPLICATION FORM SUBMISSION: Submission of the fully filled Sample Access Request form and Letter of Intent to Member Secretary of GACC.
- Step 3: UNIQUE ID GENERATION AND INTERNAL APPLICATION REVIEW FOR DOCUMENT VERIFICATION: UID will be provided to each requester after internal review for sample/ data availability along with and document verification (IBSC/IEC/Funding detail).
- Step 4: GARBH-Ini ACCESS CONTROL COMMITTEE (GACC) REVIEW: Peer review of the proposal based on scientific merit, usefulness in terms of public health importance, feasibility, appropriate use, ethical appropriateness and novelty of the proposal.
- Step 5: APPLICATION APPROVAL BY GACC: Approval of the proposal. User will be notified about the approval status through email. In case of the rejection of the proposal, user will be requested to submit the revised proposal within a year. If the user failed to submit the revised proposal, submitted annual scientific and technical support fee will not be returned.
- Step 6: MATERIAL/ DATA TRANSFER AAGREEMENT (MTA/DTA): User notification for MTA / DTA by BRF team, preparation of agreement between THST and requester.
- Step 7: SAMPLE AND DATA SHARING: User have to make their own arrangement for sample shipment following the regulatory guidelines, Data will be shared on the registered e-mail ID of the users.