Explore our Data
Data Overview
GARBH-INi is one of South East Asia's largest pregnancy cohort studies, designed to generate critical insights into maternal and child health. By collecting detailed clinical, epidemiological, and biospecimen data across multiple time points during pregnancy and postpartum, GARBH-INi supports groundbreaking research to improve maternal and neonatal outcomes.
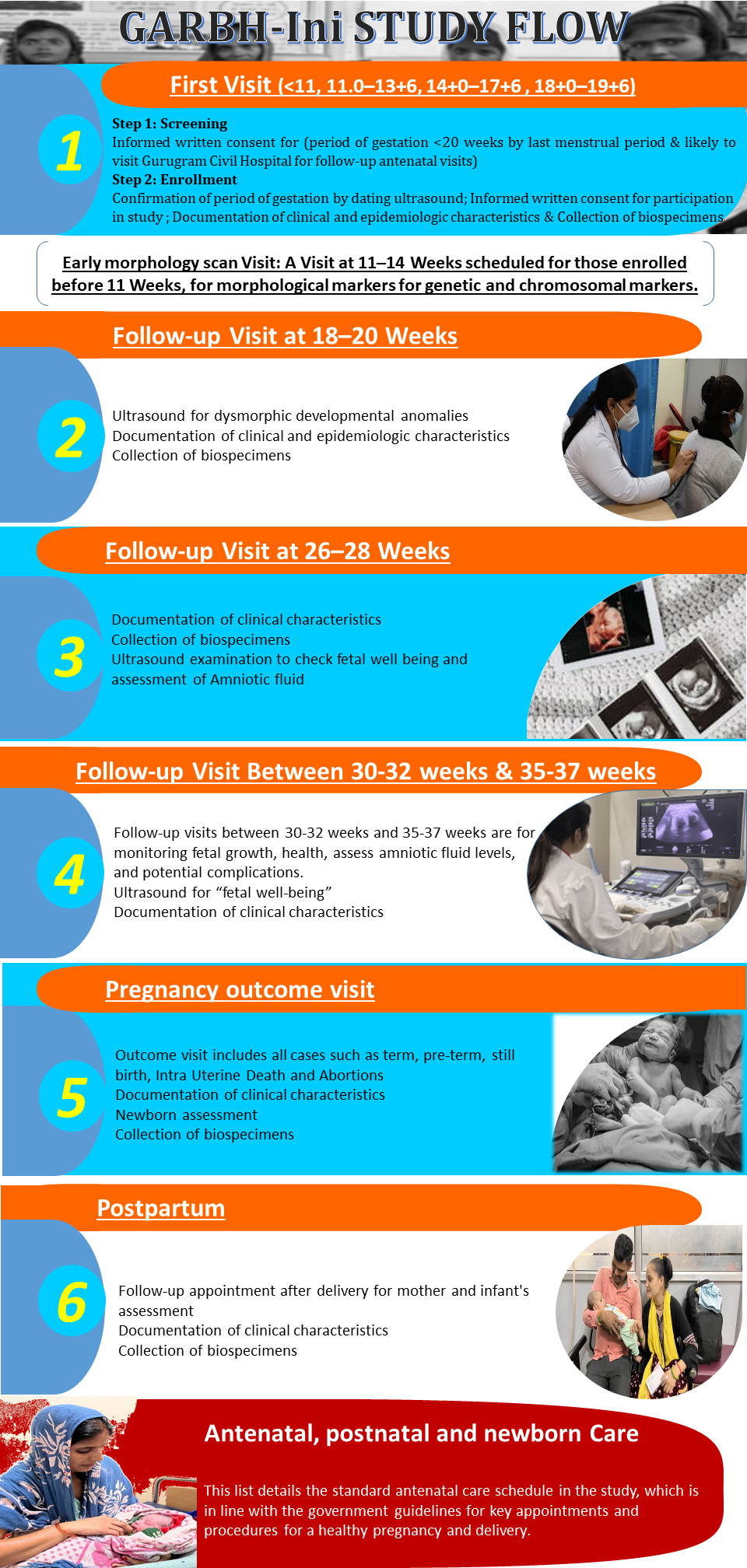
Screening:
Inclusion Criteria
- Provides verbal consent for the initial interview.
- Is willing to visit the study site during the scheduled study time points for the entire study duration.
- The participant's period of gestation, as per the last menstrual period (LMP), is ≤19 weeks and 6 days.
- Provides written informed consent to participate in the study.
Enrolment
Inclusion Criteria
- Ultrasound confirmed intrauterine pregnancy with gestational age <20 weeks
- Willing to visit the study site during the scheduled times for the complete study duration
- Written informed consent provided for participation in the study
Participants enrolled in the GARBH-INi cohort undergo detailed assessments during the following time points:
- Enrolment (≤19 weeks and 6 days gestation): Comprehensive Sociodemographic, clinical and epidemiological data collection begins.
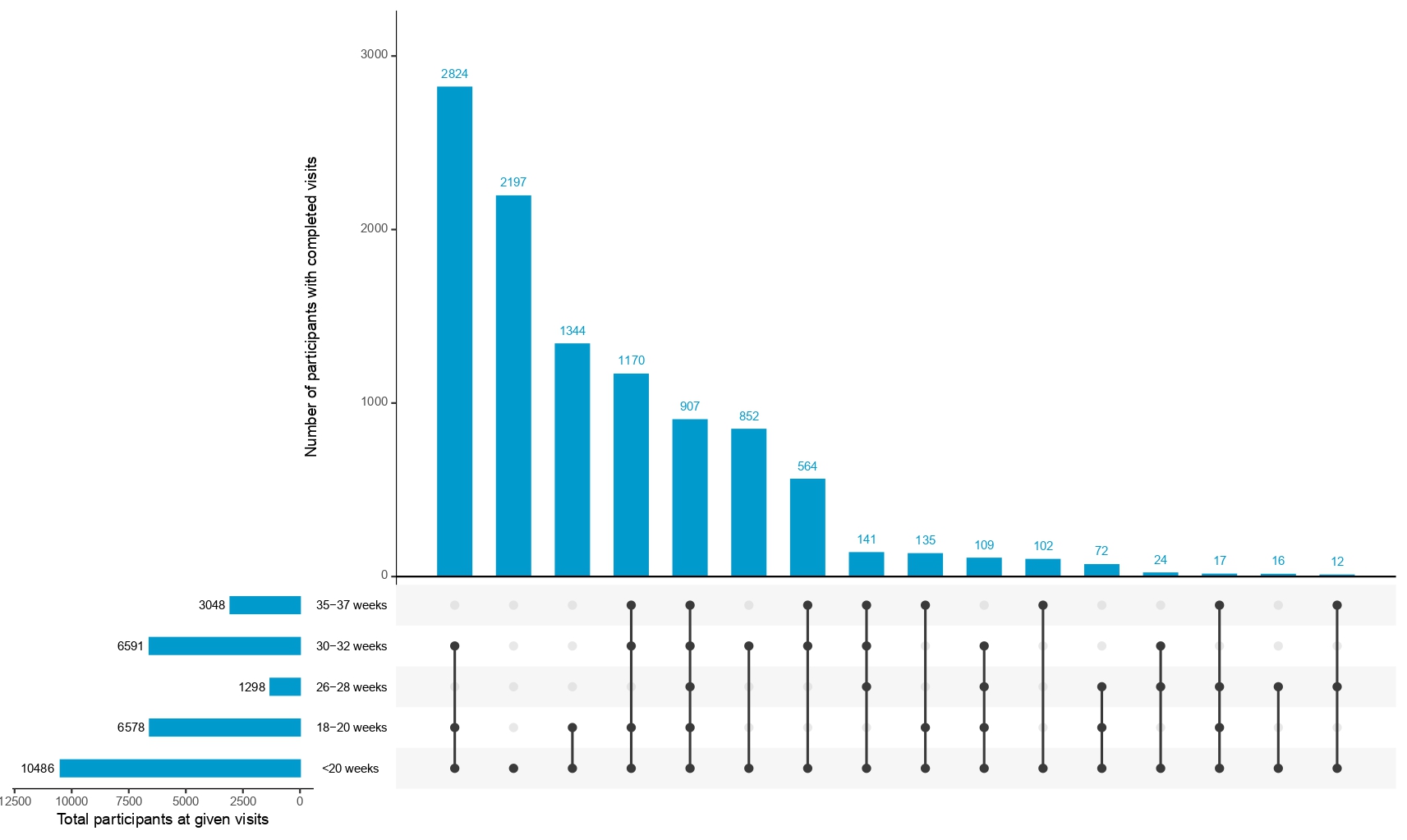
- Subsequent Visits (18-20, 26-28, 30-32, and 35-37 weeks): Clinical and epidemiological data, including biospecimens and ultrasounds, ensures continuous monitoring and detailed profiling
- Delivery: Data and biospecimens are collected at the time of childbirth.
- Postpartum: Follow-up assessments continue to capture critical maternal and neonatal health outcomes.
Clinical variables
Clinical data encompassed baseline characteristics, maternal medical history, gestational parameters, biometric measures, and trimester-specific indicators such as hemoglobin levels and blood pressure, offering an in-depth view of maternal health during pregnancy. Fetal growth metrics and maternal complications were tracked longitudinally during antenatal follow-ups, providing detailed insights into pregnancy progression.
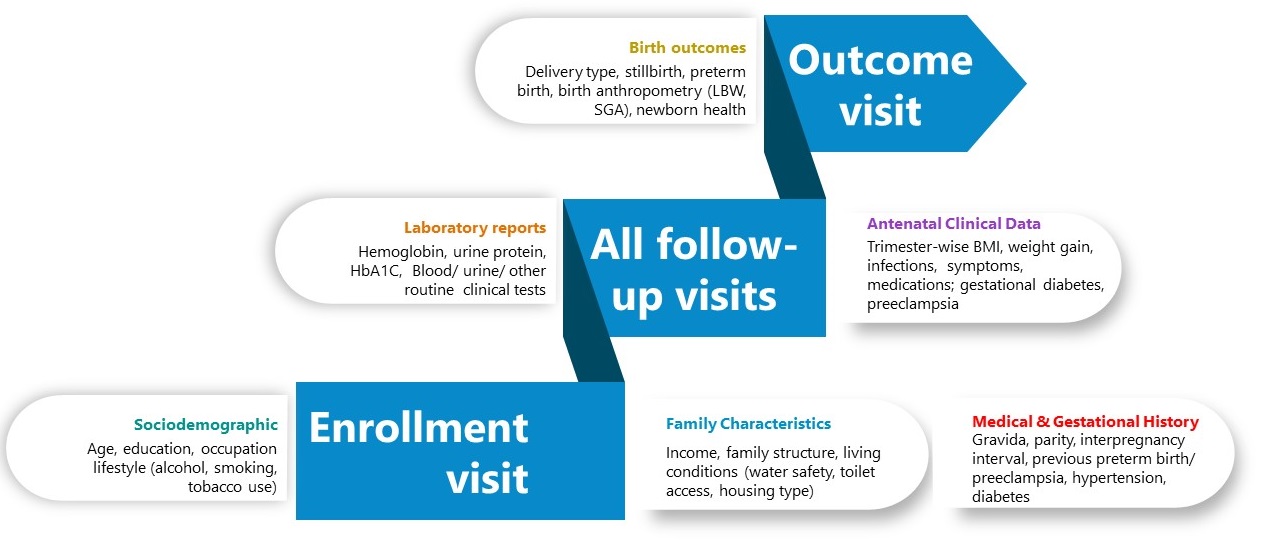
Participants attended 3–4 scheduled antenatal follow-ups (at 11–14, 18–20, 26–28, 30–32, and 35–37 weeks) and a postnatal visit between 6 weeks and 6 months postpartum. During these visits, anthropometry, physiological parameters, and pregnancy-related medical conditions, including infections and pathological events, were meticulously recorded. Birth outcomes, such as preterm birth subtypes, birth weight, stillbirths, and delivery type, were systematically documented to evaluate neonatal health.
The study implemented comprehensive quality control and standardization measures to ensure the accuracy, reliability, and consistency of data collection. The study coordinator regularly trains the research team so that inter- and intra observer variabilities are minimized. The Internal Quality Improvement (IQI) team conducts concurrent monitoring for logical, measurement, and transcription errors in the case report forms. It undertakes weekly process monitoring of maternal and neonatal anthropometry and standardization of study equipment. Research physicians address errors within a short period. Refresher trainings, calibration, and standardization exercises are executed for the reported deviations.
Clinical and Epidemiological Data: Detailed participant data, including demographic, clinical, and health records, are collected at each visit.

Ultrasound scan variables
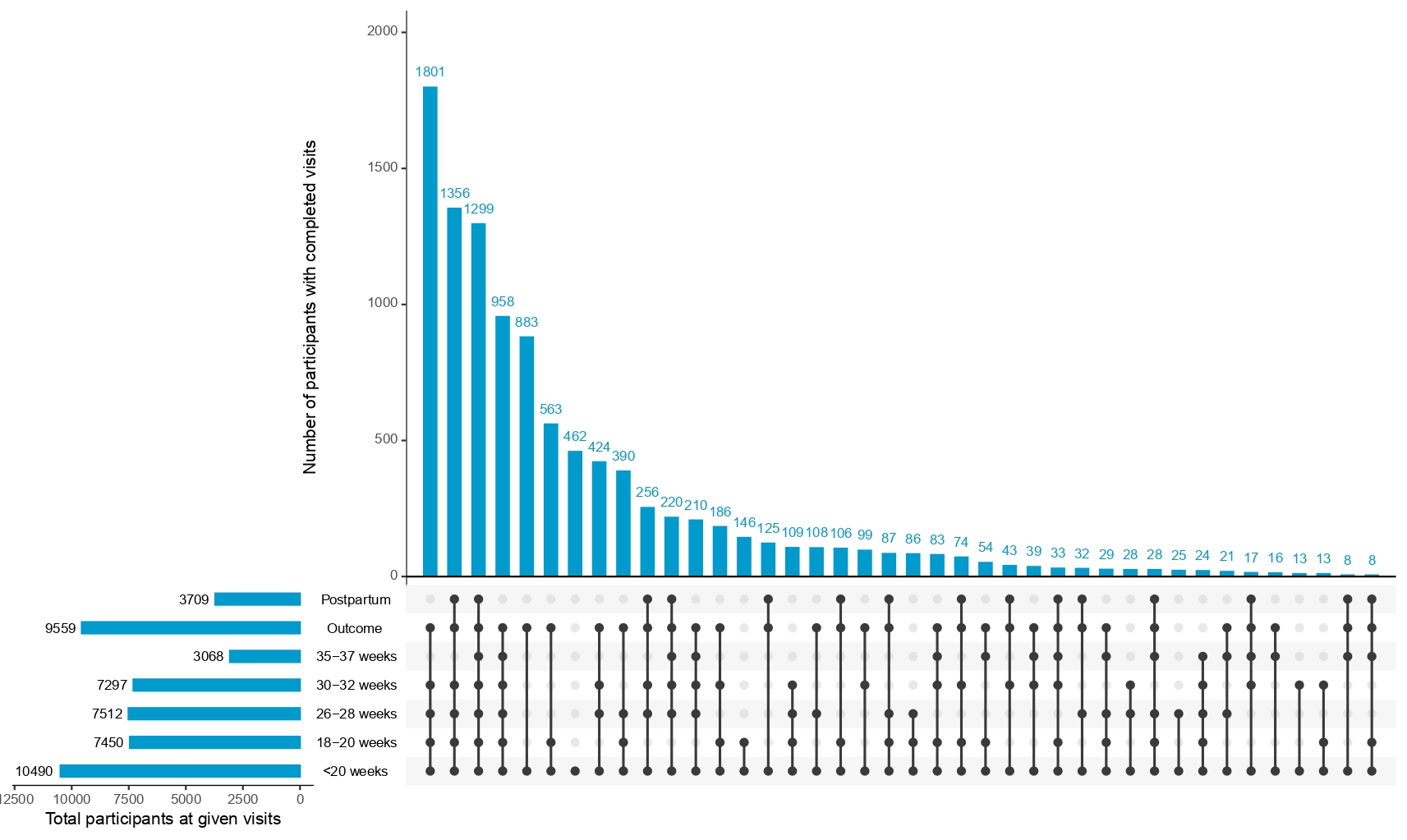
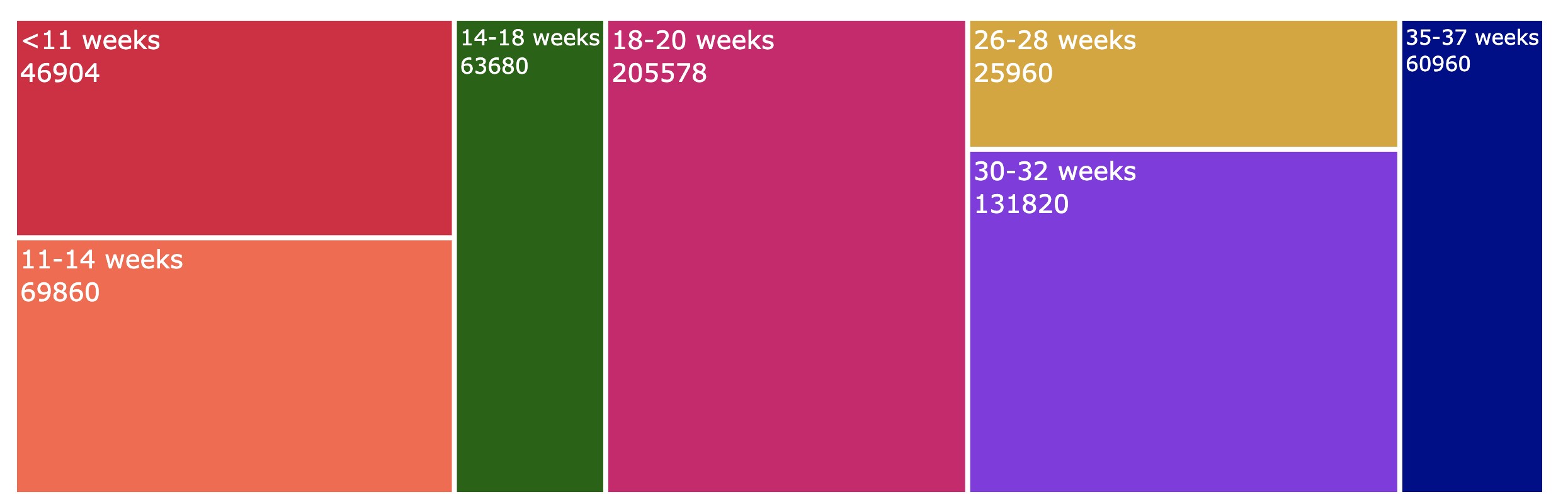
Our study has a repository of a substantial dataset with a diverse group of participants across various gestational ages. For gestational age† ≤ 11 weeks, from 4262 participants, we have collected 46882. There were a total of 4975 participants and 69,650 images between 11–14 weeks. For the 14–18 weeks range, we had 3,165 individuals and 63,300 images. Week 18–20 of gestation had the highest amount of participants at 6853 participants and 185031 images. By week 26–28, 1,298 users had uploaded 25,960 images. At 30–32 weeks, 6,585 subjects participated, generating 131,700 images. In 35–37 weeks, 3,036 individuals and 60,720 images were collected. Generating this massive volume of clinical data from pregnant females provides scope and depth of study that has not been done elsewhere in terms of providing critical fetal information to fetal well-being longitudinally
Serial images are taken by a dedicated study radiologist on a GE Voluson E8 Expert (General Electric Healthcare, Chicago, Illinois) ultrasound machine.
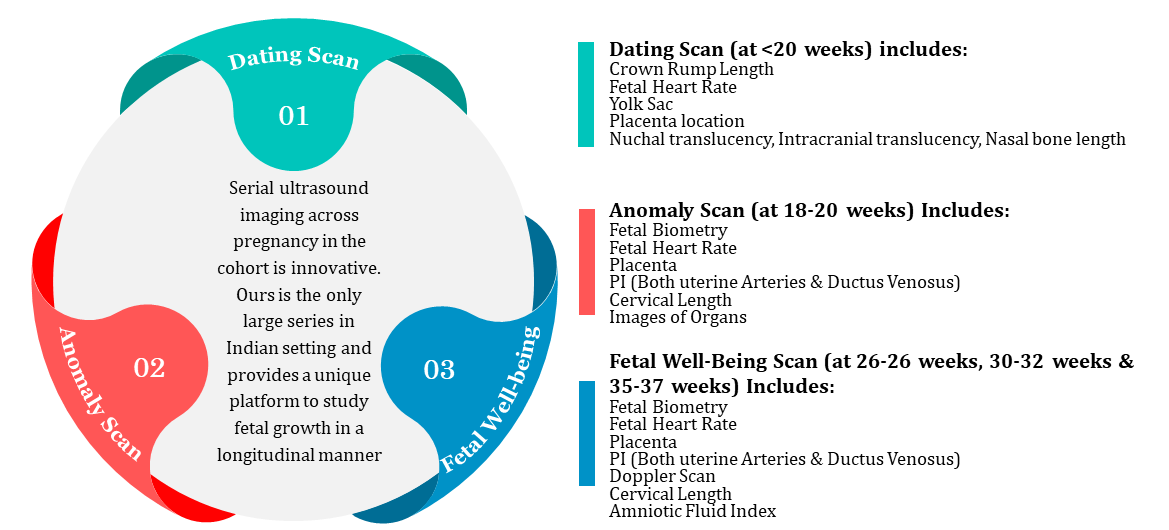
POG, fetal growth & wellbeing, placental location & morphology, and morphological markers for genetic and chromosomal abnormalities at 11–14 weeks and dysmorphic developmental anomalies at 18–20 weeks are assessed by abdominal scans. Serial changes in cervical length are measured with transvaginal scans. Doppler scans scheduled between 26-28 weeks, 30 and 32 weeks and 35-37 weeks POG measure uterine and umbilical artery Pulsatility indices and amniotic fluid index as markers of fetal wellbeing; fetal middle cerebral artery and ductus venosus blood flow are measured as potential markers of fetal growth restriction
We ensure quality control through standardized protocols, with images reviewed monthly by experienced radiologists. The study radiologist is retrained if images fall below set quality standards.
<11 weeks, 11-14 weeks, 14-18 weeks, 18-20 weeks, 26-28 weeks, 30-32 weeks, 35-37 weeks
Biospecimen Collection: Blood, urine, and saliva samples provide rich biological data for analysis.

Biospecimens
Established in 2015 under DBT supported GARBH-Ini cohort study, the facility is dedicated to the systematic collection, processing and storage of biospecimen of enrolled mothers to facilitate development of a multidimensional risk-prediction algorithm for preterm birth. The establishment of this repository was to create a national resource that would support future research in maternal and child health. As the first DBT supported biobank accredited with ISO 20387: 2018 standards, the facility adheres to internationally recognized best practices in biobanking. Additionally, it is a proud member of the International Society for Biological & Environmental Repositories (ISBER).
The Biorepository is driven by a dedicated and multidisciplinary core team comprising scientists, a quality control and quality assurance manager, data manager, technical officers, and skilled technicians. With extensive expertise in sample processing and archival, the team ensures seamless operations by adhering to rigorous protocols and internationally recognized standards, including ISO 20387:2018. Utilizing a sophisticated Laboratory Information Management System (LIMS), the team efficiently manages sample tracking, data handling, and retrieval. Regular quality checks and audits are conducted to uphold the highest standards of preservation and data accuracy.
- Established a robust biorepository to support research on preterm birth, maternal infections, fetal growth restrictions and other pregnancy conplications and adverse birth outcomes, managing over 1.4 million biospecimens from the GARBH-Ini cohort.
- Developed real-time dashboards and automated workflows for efficient data management.
- Designed pre-pandemic sera/plasma panels that were utilized as a control samples during COVID-19 Pandemic research activities.
- Supported 17 studies by providing access to a diverse and meticulously archived collection of biospecimens.
- Contributed to > 10 publications by providing access to well-preserved, high-quality biospecimens.
- Supported 12 PhD theses by providing access to high-quality biospecimens & associated metadata.
- Conducts specialized training initiatives to empower the biobanking community.
- Accredited to ISO 20387:2018, maintains a robust quality management system.
- Actively engages with ISBER and participates in global proficiency testing programs.
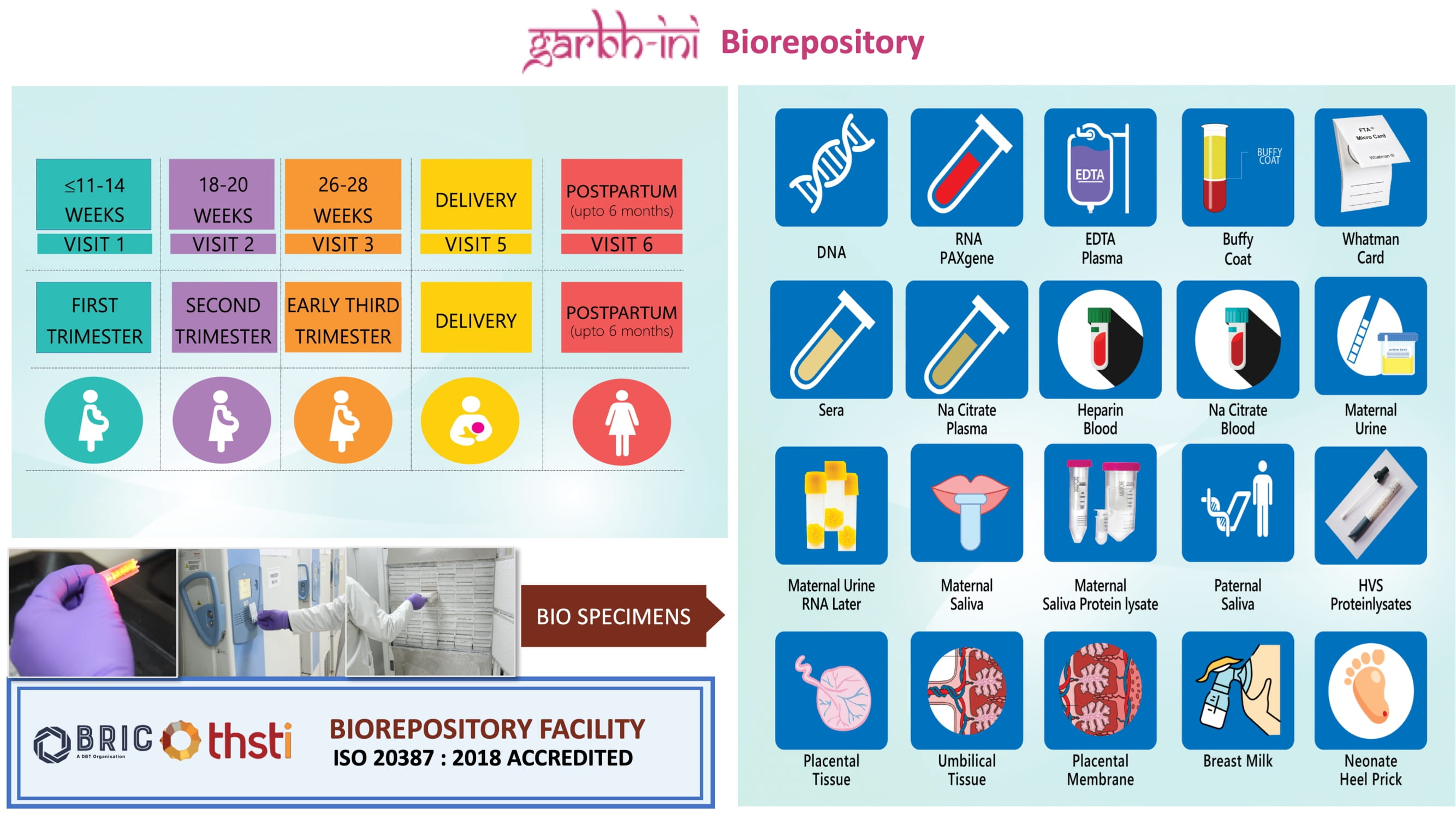
Click here for more information on biospecimens collected
First DBT supported biobank accredited with international standard ISO 20387:2018 'Biotechnology–Biobanking- General Requirements for biobanking

OMICS Data
Coordination for submission of genomics and proteomics data with IBDC is ongoing
Link to GWAS catalogue login page given below
- https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216906 for the study accession number GSE216906
- https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169338 for the study accession number GSE169338
- https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169338 for the study accession number GSE234190
